
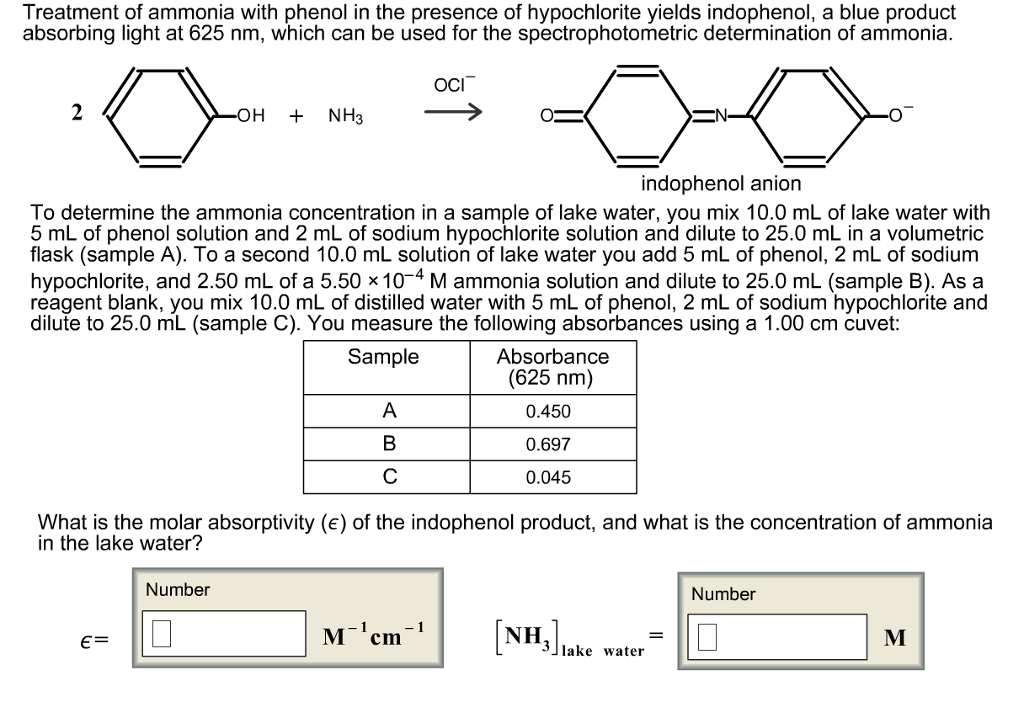
Procedure:- 1st I prepared solutions – Nessler reagent, EDTA, Ammonium sulphate,ġ0gm HgCl2 (mercuric chloride), 7gm KI(potassium iodide), 16gm NaOH (sodium Hydroxide) are dissolved in 100ml distilled water. I studied different method for estimation of ammonia in water and organic compound but here I selected one of the method for estimation of ammonia are as follow,ĭiagrammatic representation of kjeldiahl set up:. I had decided to work on this project,because fish mortality rate is gradually increases in aquaponics of vigyan ashram due to increase ammonia level in water.so I choose to do this project due to need of estimation of ammonia to solve this problem.Īim:- Estimation of percentage of ammonia by using kjeldahl process. Ammonia is excreted by animals and produced during decomposition of plants and animals, thus returning nitrogen to the aquatic system.
If phosphate is not abundant it may limit algal growth rather than nitrogen. Nitrogen can be an important factor controlling algal growth when other nutrients, such as phosphate, are abundant.

Nitrate predominates in unpolluted waters.
Nitrate and ammonia are the most common forms of nitrogen in aquatic systems. Ammonia can be converted to nitrite (NO2 ) and nitrate (NO3) by bacteria, and then used by plants. The neutral, un- ionized form (NH3 ) is highly toxic to fish and other aquatic life.Īmmonia is the preferred nitrogen-containing nutrient for plant growth. Total ammonia is what is measured analytically in water.Īmmonia is also one of the most important pollutants because it is relatively common but can be toxic, causing lower reproduction and growth, or death. Total ammonia is the sum of both NH3 and NH4 +. Its chemical formula is NH3 in the un-ionized state and NH4 + in the ionized form. Ammonia is a nutrient that contains nitrogen and hydrogen.


 0 kommentar(er)
0 kommentar(er)
